Heat of reaction for, CO(g) + 1/2 O2(g)→ CO2(g)at constant V is 67.71 K cal at 17^° C. The heat of reaction at constant P at 17^° C is
Heat of reaction for, CO(g) + 1/2 O2(g)→ CO2(g)at constant V is 67.71 K cal at 17^° C. The heat of reaction at constant P at 17^° C is
Heat of reaction for- CO-g- - 1-2 O2-g- CO2-g-at constant V is-67-71 K cal at 17- C- The heat of reaction at constant P at 17- C is

For CaCO3( s)→CaO(s)+CO2( g) at 977∘C, ΔH=174 kJ/mol; then ΔE is :-..
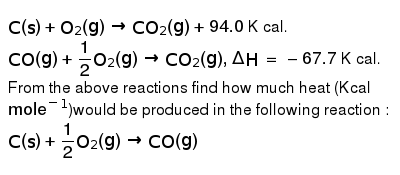
C(s)+O(2)(g)rarr CO(2)(g)+94.0 K cal. CO(g)+(1)/(2)O(2)(g)rarr CO(2)
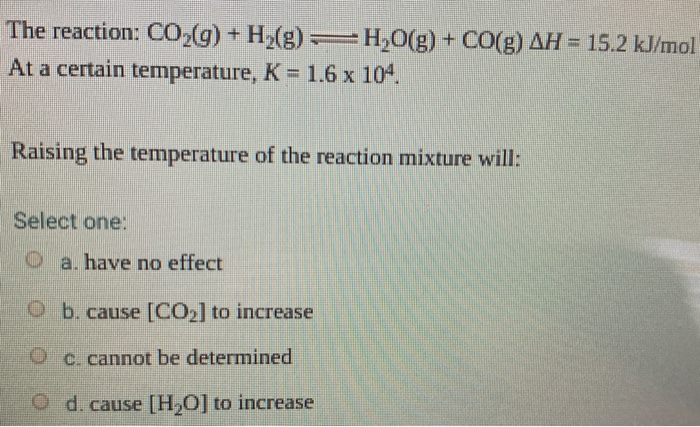
Solved The reaction: CO2(g) + H2(g) = H2O(g) + CO(g) AH =
Heat of reaction for, CO(g)+1/2O2( g)→CO2( g) at constant V is −67.71 K..
Heat of reaction for, CO(g)+1/2O2( g)→CO2( g) at constant V is −67.71 K..
(174).jpg)
Thermodynamics Exam For 12th Grade! Quiz - Trivia & Questions
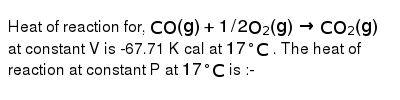
Heat of reaction for, CO(g)+1//2O(2)(g)rarr CO(2)(g) at constant V is

Heat of reaction . CO(g) + 1/O2(g) → CO2(g) constant V is-67.71 K 17℃ The heat of reaction constant P 17°C is (1-68 K a 2. 1S- (91
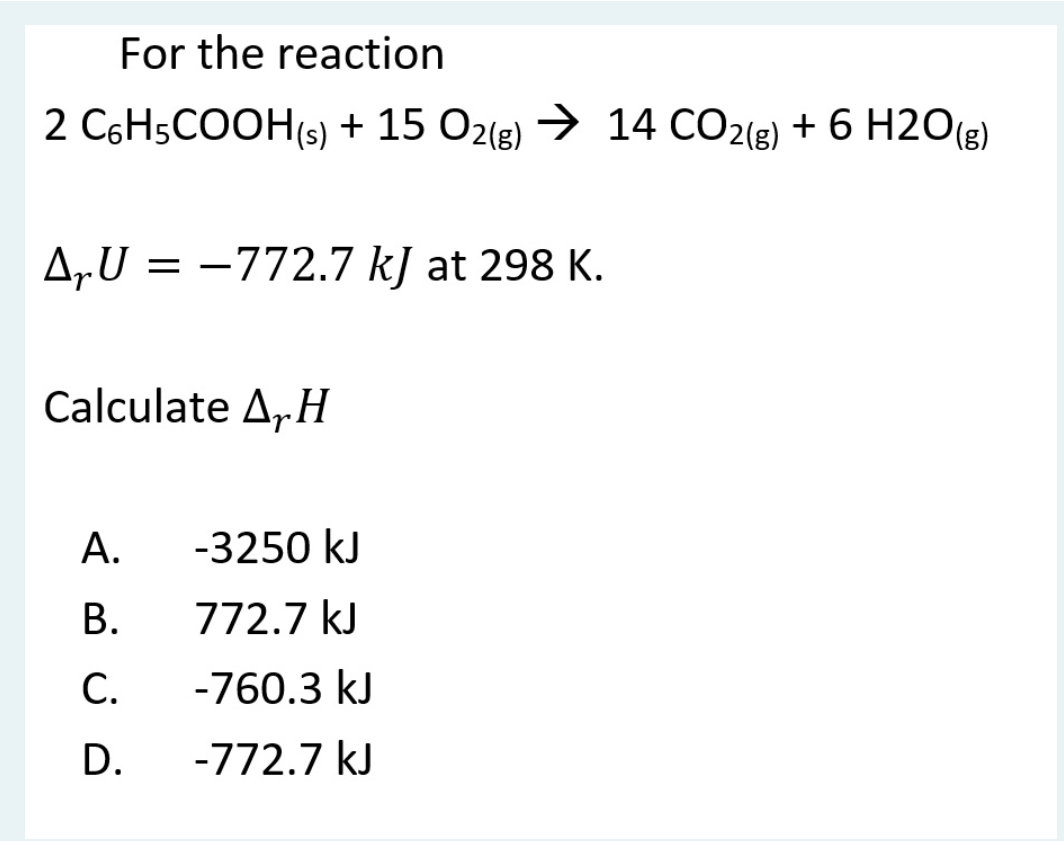
Solved For the reaction 2 C6H5COOH(s) + 15 O2(g) → 14 CO2(g)

Heat of reaction for, `CO(g)+1//2O_(2)(g)rarr CO_(2)(g)` at constant V is -67.71 K cal at `17^(@)C`
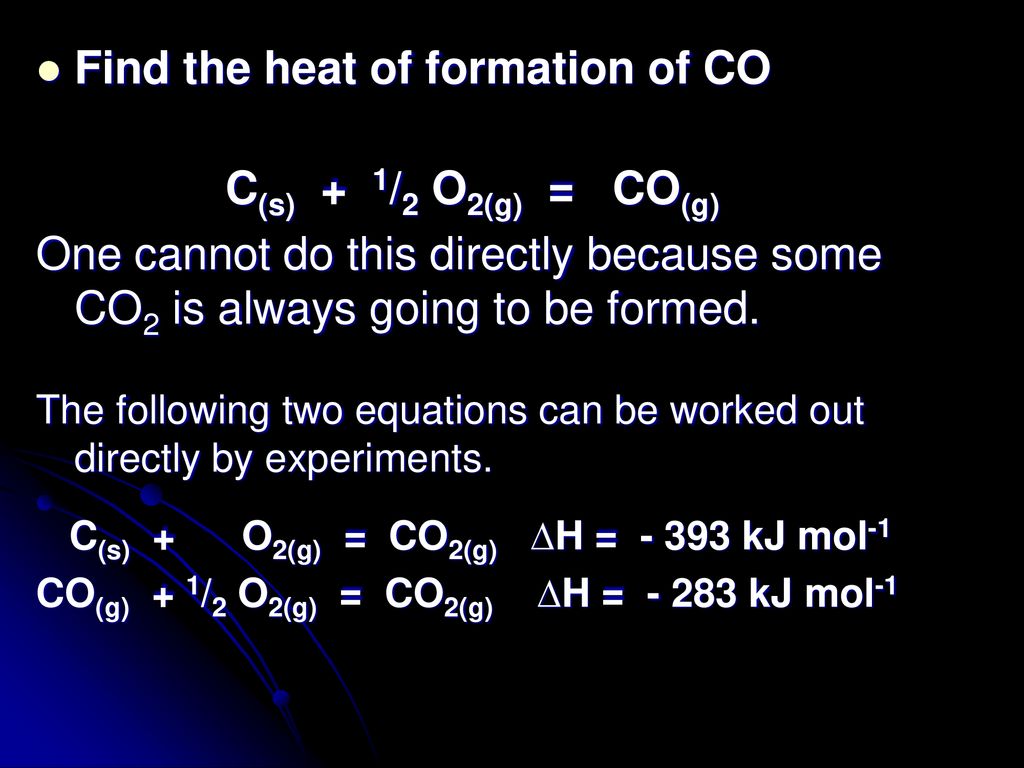
5.4.6 Hess's Law of Heat Summation [1840] - ppt download









