5.85g of NaCl is dissolved in 1L of pure water. The number of ions
5.85g of NaCl is dissolved in 1L of pure water. The number of ions in 1ml of this solution is
Vasantha, Author at WBBSE Solutions
5.85g of NaCl is dissolved in 200ml of water. What will be the molarity of the solution? - Quora
5.85g of NaCl is dissolved in 200ml of water. What will be the molarity of the solution? - Quora
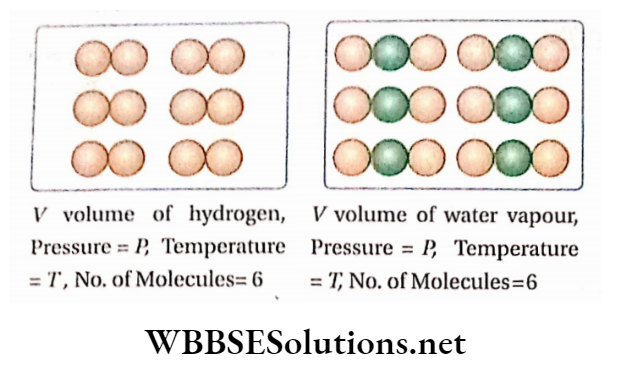
NCERT Ebook for Some Basic Concepts Of Chemistry - Some Basic Concepts Of Chemistry - Chapter 1 - NCERT Chemistry - XI

PPT - Solutions PowerPoint Presentation, free download - ID:528392

What will be the molarity of a solution, which contains 5.85 g of NaCl s per 500 mL?

FINAL fiit-jee SOME BASIC CONCEPT..docx

Class XII Solutions

Q55: In one litre of pure water, 44.4 g of calcium chloride is dissolved. The number of ions in one mL of the resultant solution is: (a) 7.23 102 (6) 7.23 1020 (C) 4.82 102 () 4.82*10** 1 Page 11 of 20
How many Na+ are present in 5.85 g of NaCl? - Quora

Molarity Calculations