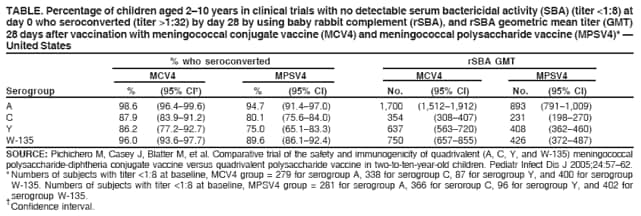
Report from the Advisory Committee on Immunization Practices (ACIP): Decision Not to Recommend Routine Vaccination of All Children Aged 2--10 Years with Quadrivalent Meningococcal Conjugate Vaccine (MCV4)

Immunization in Special Populations - Advances in Pediatrics
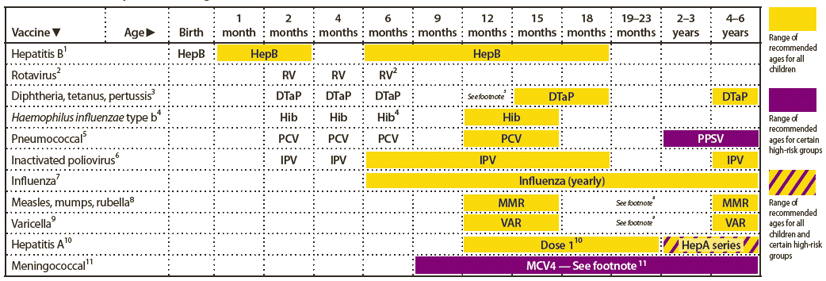
Recommended Immunization Schedules for Persons Aged 0 Through 18 Years — United States, 2012

Public health perspective of a pentavalent meningococcal vaccine combining antigens of MenACWY-CRM and 4CMenB - ScienceDirect
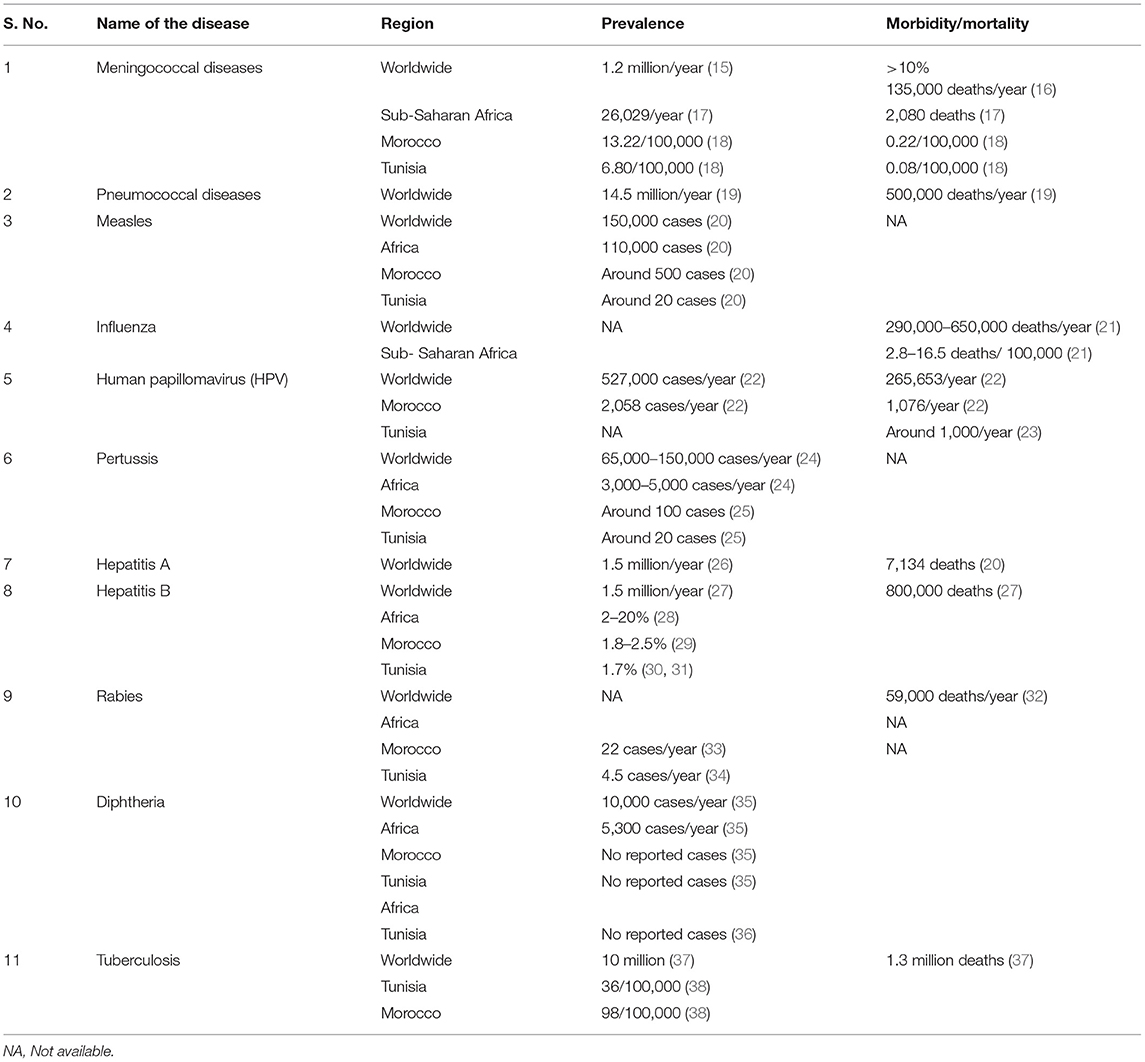
Frontiers Trends in Adult and Elderly Vaccination: Focus on Vaccination Practices in Tunisia and Morocco

Protecting the World Against Meningitis: New Recommendations From the CDC's Advisory Committee on Immunization Practices

Pediatric Immunization: Meeting the Challenges of the 21st Century
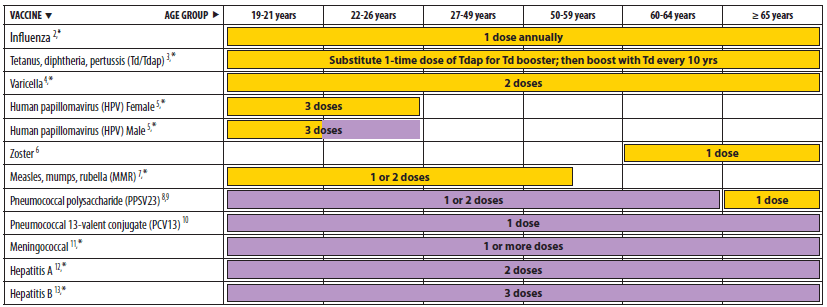
Advisory Committee on Immunization Practices (ACIP) Recommended Immunization Schedule for Adults Aged 19 Years and Older — United States, 2013

Meningococcal Disease, Mpox Added to Immunization Schedules

Core Concepts - Immunizations in Adults - Basic HIV Primary Care - National HIV Curriculum
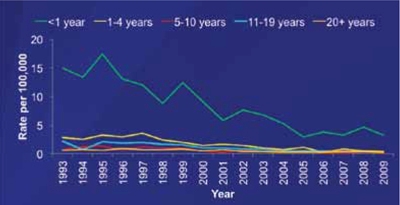
Meningococcal vaccine for infants?

Immunization schedule of the Spanish Association of Pediatrics: 2012 recommendations - ScienceDirect

Appendix A: 2012 Advisory Committee on Immunization Practices' Recommended Immunization Schedule for Children, The Childhood Immunization Schedule and Safety: Stakeholder Concerns, Scientific Evidence, and Future Studies

Biomedicines, Free Full-Text








